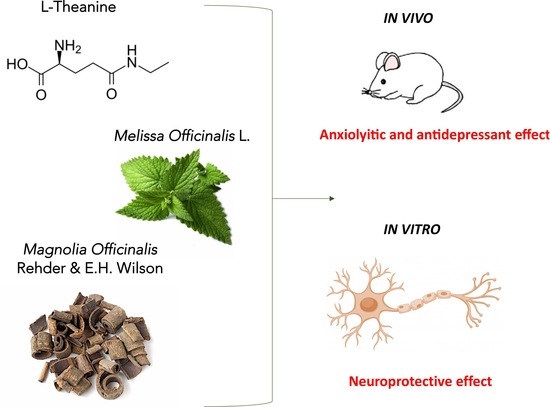
Novel Therapeutic Approach for the Management of Mood Disorders: In Vivo and In Vitro Effect of a Combination of L-Theanine, Melissa officinalis L. and Magnolia officinalis Rehder & E.H. Wilson
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemical and Drug Administration
2.3. Locomotor Activity
2.3.1. Rotarod Test
2.3.2. Hole-Board Test
2.3.3. Hot Plate Test
2.4. Anxiolytic-Like Activity
2.4.1. Light-Dark Box
2.4.2. Marbles Test
2.4.3. Novelty Suppressed Feeding Test
2.5. Antidepressant-Like Activity
Tail Suspension Test
2.6. Cell Culture
2.7. Cell Treatments
2.8. Cell Viability
2.9. Non-Competitive Sandwich ELISA Protocol for BDNF
2.10. Data and Statistical Analysis
3. Results
3.1. Anxiolytic Effect of TMM
3.2. Antidepressant-Like Activity in a Depressant-Like Paradigm
3.3. Lack of Impairment of Locomotor Behaviour and Hypersensibilization to Thermal Stimulus
3.4. Neuroprotective Effect of TMM on SH-SY5Y Neuronal Cells
3.5. Involvement of Endocannabinoid System in the Final Effect of TMM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nemeroff, C.B.; Owens, M.J. Treatment of mood disorders. Nat. Neurosci. 2002, 5, 1068–1070. [Google Scholar] [CrossRef]
- Kimball, S.M.; Mirhosseini, N.; Rucklidge, J. Database Analysis of Depression and Anxiety in a Community Sample-Response to a Micronutrient Intervention. Nutrients 2018, 10, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, K.S.; Hernandez, M.; Mao, J.J.; Haviland, I.; Gubili, J. Herbal medicine for depression and anxiety: A systematic review with assessment of potential psycho-oncologic relevance. Phyther. Res. 2018, 32, 865–891. [Google Scholar] [CrossRef]
- Tiller, J.W.G. Depression and anxiety. Med. J. Aust. 2013, 199, S28–S31. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Byrne, G.J.; Cribb, L.; Oliver, G.; Murphy, J.; Macdonald, P.; Nazareth, S.; Karamacoska, D.; Galea, S.; Short, A.; et al. L-theanine in the adjunctive treatment of generalized anxiety disorder: A double-blind, randomised, placebo-controlled trial. J. Psychiatr. Res. 2019, 110, 31–37. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Available online: https://www.who.int (accessed on 16 June 2020).
- European Medicine Agency. Available online: https://www.ema.europa.eu (accessed on 16 June 2020).
- Yang, C.S.; Chen, G.; Wu, Q. Recent scientific studies of a traditional Chinese medicine, tea, on prevention of chronic diseases. J. Tradit. Complement. Med. 2014, 4, 17–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, J.L.; Everett, J.M.; D’Cunha, N.M.; Sergi, D.; Georgousopoulou, E.N.; Keegan, R.J.; McKune, A.J.; Mellor, D.D.; Anstice, N.; Naumovski, N. The Effects of Green Tea Amino Acid L-Theanine Consumption on the Ability to Manage Stress and Anxiety Levels: A Systematic Review. Plant Foods Hum. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zukhurova, M.; Prosvirnina, M.; Daineko, A.; Simanenkova, A.; Petrishchev, N.; Sonin, D.; Galagudza, M.; Shamtsyan, M.; Juneja, L.R.; Vlasov, T. L-theanine administration results in neuroprotection and prevents glutamate receptor agonist-mediated injury in the rat model of cerebral ischemia-reperfusion. Phyther. Res. 2013, 27, 1282–1287. [Google Scholar] [CrossRef]
- Kakuda, T.; Hinoi, E.; Abe, A.; Nozawa, A.; Ogura, M.; Yoneda, Y. Theanine, an ingredient of green tea, inhibits [3H] glutamine transport in neurons and astroglia in rat brain. J. Neurosci. Res. 2008, 86, 1846–1856. [Google Scholar] [CrossRef]
- Bergink, V.; van Megen, H.J.G.M.; Westenberg, H.G.M. Glutamate and anxiety. Eur. Neuropsychopharmacol. 2004, 14, 175–183. [Google Scholar] [CrossRef]
- Borgonetti, V.; Governa, P.; Montopoli, M.; Biagi, M. Cannabis sativa L. Constituents and Their Role in Neuroinflammation. Curr. Bioact. Compd. 2019, 15, 147–158. [Google Scholar] [CrossRef]
- Sánchez-Blázquez, P.; Rodríguez-Muñoz, M.; Garzón, J. The cannabinoid receptor 1 associates with NMDA receptors to produce glutamatergic hypofunction: Implications in psychosis and schizophrenia. Front. Pharmacol. 2014, 4, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiarlone, A.; Bellocchio, L.; Blázquez, C.; Resel, E.; Soria-Gómez, E.; Cannich, A.; Ferrero, J.J.; Sagredo, O.; Benito, C.; Romero, J.; et al. A restricted population of CB1 cannabinoid receptors with neuroprotective activity. Proc. Natl. Acad. Sci. USA 2014, 111, 8257–8262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruyama, Y.; Kuribara, H.; Morita, M.; Yuzurihara, M.; Weintraub, S.T. Identification of magnolol and honokiol as anxiolytic agents in extracts of Saiboku-to, an oriental herbal medicine. J. Nat. Prod. 1998, 61, 135–138. [Google Scholar] [CrossRef]
- World Health Organization. WHO Monographs on Selected Medicinal Plants Volume 4; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Rempel, V.; Fuchs, A.; Hinz, S.; Karcz, T.; Lehr, M.; Koetter, U.; Müller, C.E. Magnolia extract, magnolol, and metabolites: Activation of cannabinoid CB2 receptors and blockade of the related GPR. ACS Med. Chem. Lett. 2013, 4, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Kalueff, A.V.; Nutt, D.J. Role of GABA in anxiety and depression. Depress. Anxiety 2007, 24, 495–517. [Google Scholar] [CrossRef]
- EMA Assessment Report on Melissa officinalis L., Folium. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-melissa-officinalis-l-folium_en.pdf (accessed on 16 June 2020).
- Shakeri, A.; Sahebkar, A.; Javadi, B. Melissa officinalis L.—A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 188, 204–228. [Google Scholar] [CrossRef]
- Sasaki, K.; El Omri, A.; Kondo, S.; Han, J.; Isoda, H. Rosmarinus officinalis polyphenols produce anti-depressant like effect through monoaminergic and cholinergic functions modulation. Behav. Brain Res. 2013, 238, 86–94. [Google Scholar] [CrossRef]
- Awad, R.; Muhammad, A.; Durst, T.; Trudeau, V.L.; Arnason, J.T. Bioassay-guided Fractionation of Lemon Balm (Melissa officinalis L.) using an In Vitro Measure of GABA Transaminase Activity. Phyther. Res. 2009, 23, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.C.; Lilley, E. Implementing guidelines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP. Br. J. Pharmacol. 2015, 172, 3189–3193. [Google Scholar] [CrossRef] [Green Version]
- Charan, J.; Kantharia, N. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef] [Green Version]
- Sanna, M.D.; Borgonetti, V.; Galeotti, N. μ Opioid Receptor-Triggered Notch-1 Activation Contributes to Morphine Tolerance: Role of Neuron—Glia Communication. Mol. Neurobiol. 2019. [Google Scholar] [CrossRef]
- Bourin, M.; Hascoet, M. The mouse light/dark box test. Eur. J. Pharmacol. 2003, 463, 55–65. [Google Scholar] [CrossRef]
- Yalcin, I.; Bohren, Y.; Waltisperger, E.; Sage-Ciocca, D.; Yin, J.C.; Freund-Mercier, M.J.; Barrot, M. A time-dependent history of mood disorders in a murine model of neuropathic pain. Biol. Psychiatry 2011, 70, 946–953. [Google Scholar] [CrossRef]
- Bodnoff, S.R.; Suranyi-Cadotte, B.; Aitken, D.H.; Quirion, R.; Meaney, M.J. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology 1988, 95, 298–302. [Google Scholar] [CrossRef]
- Galeotti, N.; Ghelardini, C. Regionally selective activation and differential regulation of ERK, JNK and p38 MAP kinase signalling pathway by protein kinase C in mood modulation. Int. J. Neuropsychopharmacol. 2012, 15, 781–793. [Google Scholar] [CrossRef] [Green Version]
- Kritis, A.A.; Stamoula, E.G.; Paniskaki, K.A.; Vavilis, T.D. Researching glutamate—Induced cytotoxicity in different cell lines: A comparative/collective analysis/study. Front. Cell. Neurosci. 2015, 9, 91. [Google Scholar] [CrossRef] [Green Version]
- Curtis, M.J.; Alexander, S.; Cirino, G.; Docherty, J.R.; George, C.H.; Giembycz, M.A.; Hoyer, D.; Insel, P.A.; Izzo, A.A.; Ji, Y.; et al. Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. Br. J. Pharmacol. 2018, 175, 987–993. [Google Scholar] [CrossRef] [Green Version]
- Sanna, M.D.; Les, F.; Lopez, V.; Galeotti, N. Lavender (Lavandula angustifolia Mill.) essential oil alleviates neuropathic pain in mice with spared nerve injury. Front. Pharmacol. 2019, 10, 472. [Google Scholar] [CrossRef]
- Barfield, E.T.; Alexandra Moser, V.; Hand, A.; Grisel, J.E. ß-Endorphin Modulates the Effect of Stress on Novelty-Suppressed Feeding. Front. Behav. Neurosci. 2013, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Pereira, V.; Goudet, C. Emerging Trends in Pain Modulation by Metabotropic Glutamate Receptors. Front. Mol. Neurosci. 2019, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Sun, L.H.; Yang, W.; Cui, R.J.; Xu, S.B. The role of BDNF in the neuroimmune axis regulation of mood disorders. Front. Neurol. 2019, 10, 515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandelow, B.; Michaelis, S.; Wedekind, D. Treatment of anxiety disorders. Dialogues Clin. Neurosci. 2017, 19, 93–107. [Google Scholar] [PubMed]
- Governa, P.; Carullo, G.; Biagi, M.; Aiello, F. Evaluation of the In Vitro Wound-Healing Activity of Calabrian Honeys. Antioxidants. 2019, 8, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiocchio, I.; Poli, F.; Governa, P.; Biagi, M.; Lianza, M. Wound healing and in vitro antiradical activity of five Sedum species grown within two sites of community importance in Emilia-Romagna (Italy). Plant Biosyst. 2019, 153, 610–615. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G.; Sarris, J. Rosenroot (Rhodiola rosea): Traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine 2010, 17, 481–493. [Google Scholar] [CrossRef]
- Galeotti, N.; Vivoli, E.; Bilia, A.R.; Vincieri, F.F.; Ghelardini, C. St. John’s Wort reduces neuropathic pain through a hypericin-mediated inhibition of the protein kinase Cgamma and epsilon activity. Biochem. Pharmacol. 2010, 79, 1327–1336. [Google Scholar] [CrossRef] [Green Version]
- Winer, E.S.; Bryant, J.; Bartoszek, G.; Rojas, E.; Nadorff, M.R.; Kilgore, J. Mapping the relationship between anxiety, anhedonia, and depression. J. Affect. Disord. 2017, 221, 289–296. [Google Scholar] [CrossRef]
- Baumeister, H.; Hutter, N.; Bengel, J. Psychological and pharmacological interventions for depression in patients with diabetes mellitus and depression. Cochrane Database Syst. Rev. 2012, 12, CD008381. [Google Scholar] [CrossRef]
- Salum, G.A.; Manfro, G.G.; Fleck, M.P. What is not “Effective” in Mild to Moderate Depression: Antidepressants or the Hamilton Rating Scale for Depression? CNS Spectr. 2011, 16, 99. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, J.M. The effects of antidepressants on sexual functioning in depressed patients: A review. J. Clin. Psychiatry 2001, 62 (Suppl. S3), 22–34. [Google Scholar]
- Hidese, S.; Ota, M.; Wakabayashi, C.; Noda, T.; Ozawa, H.; Okubo, T.; Kunugi, I. Effects of chronic l-theanine administration in patients with major depressive disorder: An open-label study. Acta Neuropsychiatr. 2017, 29, 72–79. [Google Scholar] [CrossRef]
- Wakabayashi, C.; Numakawa, T.; Ninomiya, M.; Chiba, S.; Kunugi, H. Behavioral and molecular evidence for psychotropic effects in L-theanine. Psychopharmacology 2012, 219, 1099–1109. [Google Scholar] [CrossRef]
- Yin, C.; Gou, L.; Liu, Y.; Yin, X.; Zhang, L.; Jia, G.; Zhuang, X. Antidepressant-like effects of L-theanine in the forced swim and tail suspension tests in mice. Phytother. Res. 2011, 25, 1636–1639. [Google Scholar] [CrossRef]
- Petrovic, M.; Mariman, A.; Warie, H.; Afschrift, M.; Pevernagie, D. Is there a rationale for prescription of benzodiazepines in the elderly? Review of the literature. Acta Clin. Belg. 2003, 58, 27–36. [Google Scholar] [CrossRef]
- Hidese, S.; Ogawa, S.; Ota, M.; Ishida, I.; Yasukawa, Z.; Ozeki, M.; Kunugi, H. Effects of L-Theanine Administration on Stress-Related Symptoms and Cognitive Functions in Healthy Adults: A Randomized Controlled Trial. Nutrients 2019, 11, 2362. [Google Scholar] [CrossRef] [Green Version]
- White, D.J.; de Klerk, S.; Woods, W.; Gondalia, S.; Noonan, C.; Scholey, A.B. Anti-stress, behavioural and magnetoencephalography effects of an l-theanine-based nutrient drink: A randomised, double-blind, placebo-controlled, crossover trial. Nutrients 2016, 8, 53. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.; Gray, M.A.; Oliver, C.; Liley, D.T.; Harrison, B.J.; Bartholomeusz, C.F.; Phan, K.L.; Nathan, P.J. The acute effects of L-theanine in comparison with alprazolam on anticipatory anxiety in humans. Hum. Psychopharmacol. 2004, 19, 457–465. [Google Scholar] [CrossRef]
- Lopes Sakamoto, F.; Metzker Pereira Ribeiro, R.; Amador Bueno, A.; Oliveira Santos, H. Psychotropic effects of L-theanine and its clinical properties: From the management of anxiety and stress to a potential use in schizophrenia. Pharmacol. Res. 2019, 147, 104395. [Google Scholar] [CrossRef]
- Kakuda, T.; Yanase, H.; Utsunomiya, K.; Nozawa, A.; Unno, T.; Kataoka, K. Protective effect of γ-glutamylethylamide (theanine) on ischemic delayed neuronal death in gerbils. Neurosci. Lett. 2000, 289, 189–192. [Google Scholar] [CrossRef]
- Ota, M.; Wakabayashi, C.; Sato, N.; Hori, H.; Hattori, K.; Teraishi, T.; Ozakawa, H.; Okubo, T.; Kunugi, H. Effect of L-theanine on glutamatergic function in patients with schizophrenia. Acta Neuropsychiatr. 2015, 27, 291–296. [Google Scholar] [CrossRef]
- Ogawa, S.; Ota, M.; Ogura, J.; kato, K.; Konugi, H. Effects of L-theanine on anxiety-like behavior, cerebrospinal fluid amino acid profile, and hippocampal activity in Wistar Kyoto rats. Psychopharmacology 2018, 235, 37–45. [Google Scholar] [CrossRef]
- Poleszak, E.; Socała, K.; Szopa, A.; Wróbel, A.; Szewczyk, B.; Kasperek, R.; Blicharska, E.; Nowak, G.; Wlaź, P. Involvement of NMDA receptor complex in the anxiolytic-like effects of chlordiazepoxide in mice. J. Neural Transm. 2011, 118, 857–864. [Google Scholar] [CrossRef] [Green Version]
- Sanacora, G.; Treccani, G.; Popoli, M. Towards a glutamate hypothesis of depression: An emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 2012, 62, 63–77. [Google Scholar] [CrossRef] [Green Version]
- Ibarra, A.; Feuillere, N.; Roller, M.; Lesburgere, E.; Beracochea, D. Effects of chronic administration of Melissa officinalis L. extract on anxiety-like reactivity and on circadian and exploratory activities in mice. Phytomedicine 2010, 17, 397–403. [Google Scholar] [CrossRef]
- Ghazizadeh, J.; Hamedeyazdan, S.; Torbati, M.; Farajdokht, F.; Fakhari, A.; Mahmoudi, J.; Araj-khodaei, M.; Sadigh-Eteghad, S. Melissa officinalis L. hydro-alcoholic extract inhibits anxiety and depression through prevention of central oxidative stress and apoptosis. Exp. Physiol. 2020, 105, 707–720. [Google Scholar] [CrossRef]
- Haybar, H.; Javid, A.Z.; Haghighizadeh, M.H.; Valizadeh, E.; Mohaghegh, S.M.; Mohammadzadeh, A. The effects of Melissa officinalis supplementation on depression, anxiety, stress, and sleep disorder in patients with chronic stable angina. Clin. Nutr. ESPEN 2018, 26, 47–52. [Google Scholar] [CrossRef]
- Rey, A.A.; Purrio, M.; Viveros, M.P.; Lutz, B. Biphasic effects of cannabinoids in anxiety responses: CB1 and GABA B receptors in the balance of gabaergic and glutamatergic neurotransmission. Neuropsychopharmacology 2012, 37, 2624–2634. [Google Scholar] [CrossRef] [Green Version]
- Talbott, S.M.; Talbott, J.A.; Pugh, M. Effect of Magnolia officinalis and Phellodendron amurense (Relora®) on cortisol and psychological mood state in moderately stressed subjects. J. Int. Soc. Sports Nutr. 2013, 10, 37. [Google Scholar] [CrossRef] [Green Version]
- Kuribara, H.; Stavinoha, W.B.; Maruyama, Y. Behavioural pharmacological characteristics of honokiol, an anxiolytic agent present in extracts of Magnolia bark, evaluated by an elevated plus-maze test in mice. J. Pharm. Pharmacol. 1998, 50, 819–826. [Google Scholar] [CrossRef]
- Niciu, M.J.; Kelmendi, B.; Sanacora, G. Overview of Glutamatergic Neurotransmission in the Nervous System. Pharmacol. Biochem. Behav. 2012, 100, 656–664. [Google Scholar] [CrossRef] [Green Version]
- Cortese, B.M.; Phan, K.L. The role of glutamate in anxiety and related disorders. CNS Spectr. 2005, 10, 820–830. [Google Scholar] [CrossRef]
- Croce, N.; Mathé, A.A.; Gelfo, F.; Caltagirone, C.; Bernardini, S.; Angelucci, F. Effects of lithium and valproic acid on BDNF protein and gene expression in an in vitro human neuron-like model of degeneration. J. Psychopharmacol. 2014, 28, 964–972. [Google Scholar] [CrossRef]
- Mancini, A.; Chelini, A.; Di Capua, A.; Castelli, L.; Brogi, S.; Paolino, M.; Giuliani, G.; Cappelli, A.; Frosini, M.; Ricci, L.; et al. Synthesis and biological evaluation of a new class of benzothiazines as neuroprotective agents. Eur. J. Med. Chem. 2017, 126, 614–630. [Google Scholar] [CrossRef]
- Hashimoto, R.; Hough, C.; Nakazawa, T.; Yamamoto, T.; Chuang, D.M. Lithium protection against glutamate excitotoxicity in rat cerebral cortical neurons: Involvement of NMDA receptor inhibition possibly by decreasing NR2B tyrosine phosphorylation. J. Neurochem. 2002, 80, 589–597. [Google Scholar] [CrossRef] [Green Version]
- Martinowich, K.; Manji, H.; Lu, B. New insights into BDNF function in depression and anxiety. Nat. Neurosci. 2007, 10, 1089–1093. [Google Scholar] [CrossRef]
- Zhong, F.; Liu, L.; Wei, J.L.; Hu, Z.L.; Li, L.; Wang, S.; Xu, J.M.; Zhou, X.F.; Li, C.Q.; Yang, Z.Y.; et al. Brain-derived neurotrophic factor precursor in the hippocampus regulates both depressive and anxiety-like behaviors in rats. Front. Psychiatry 2019, 9, 776. [Google Scholar] [CrossRef] [Green Version]
- Phillips, C. Brain-Derived Neurotrophic Factor, Depression, and Physical Activity: Making the Neuroplastic Connection. Neural Plast. 2017, 2017, 7260130. [Google Scholar] [CrossRef]
- Finsterwald, C.; Fiumelli, H.; Cardinaux, J.R.; Martin, J.L. Regulation of dendritic development by BDNF requires activation of CRTC1 by glutamate. J. Biol. Chem. 2010, 285, 28587–28595. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.L.; Finsterwald, C. Cooperation between BDNF and glutamate in the regulation of synaptic transmission and neuronal development. Commun. Integr. Biol. 2011, 4, 14–16. [Google Scholar] [CrossRef]
- Mattson, M.P. Excitotoxic and excitoprotective mechanisms: Abundant targets for the prevention and treatment of neurodegenerative disorders. NeuroMolecular Med. 2003, 3, 65–94. [Google Scholar] [CrossRef] [Green Version]
- Mattson, M.P. Glutamate and Neurotrophic Factors in Neuronal Plasticity and Disease. Ann. N. Y. Acad. Sci. 2009, 1144, 97–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Iglesia-Larrad, J.I.; Barral, C.; Casado-Espada, N.M.; de Alarcón, R.; Maciá-Casas, A.; Vicente Hernandez, B.; Roncero, C. Benzodiazepine abuse, misuse, dependence, and withdrawal among schizophrenic patients: A review of the literature. Psychiatry Res. 2020, 284, 112660. [Google Scholar] [CrossRef] [PubMed]
- Lader, M.; Kyriacou, A. Withdrawing Benzodiazepines in Patients With Anxiety Disorders. Curr. Psychiatry Rep. 2016, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Baur, R.; Gertsch, J.; Sigel, E. The cannabinoid CB 1receptor antagonists rimonabant (SR141716) and AM251 directly potentiate GABA A receptors. Br. J. Pharmacol. 2012, 165, 2479–2484. [Google Scholar] [CrossRef] [PubMed] [Green Version]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borgonetti, V.; Governa, P.; Biagi, M.; Galeotti, N. Novel Therapeutic Approach for the Management of Mood Disorders: In Vivo and In Vitro Effect of a Combination of L-Theanine, Melissa officinalis L. and Magnolia officinalis Rehder & E.H. Wilson. Nutrients 2020, 12, 1803. https://doi.org/10.3390/nu12061803
Borgonetti V, Governa P, Biagi M, Galeotti N. Novel Therapeutic Approach for the Management of Mood Disorders: In Vivo and In Vitro Effect of a Combination of L-Theanine, Melissa officinalis L. and Magnolia officinalis Rehder & E.H. Wilson. Nutrients. 2020; 12(6):1803. https://doi.org/10.3390/nu12061803
Chicago/Turabian StyleBorgonetti, Vittoria, Paolo Governa, Marco Biagi, and Nicoletta Galeotti. 2020. "Novel Therapeutic Approach for the Management of Mood Disorders: In Vivo and In Vitro Effect of a Combination of L-Theanine, Melissa officinalis L. and Magnolia officinalis Rehder & E.H. Wilson" Nutrients 12, no. 6: 1803. https://doi.org/10.3390/nu12061803