Abstract
Drugs can cause obvious damage to the brain. To verify the relationship between acupuncture, neurotrophic factor expression and brain cell structural changes, this study established a rat model of heroin relapse using intramuscular injection of increasing amounts of heroin. During the detoxification period, rat models received acupuncture at Baihui (DU20) and Dazhui (DU14). Electron microscopy demonstrated that the structure of the ventral tegmental area in heroin relapse rats gradually became normalized after acupuncture treatment. Immunohistochemical staining exhibited that the expression of brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor increased in the ventral tegmental area following acupuncture. Moreover, the effects were similar to that of methadone, a type of medicine called an opioid. Results suggested that acupuncture at Baihui and Dazhui protected brain neurons against injury in rats with heroin relapse by promoting brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor expression.
Keywords: nerve regeneration, acupuncture and moxibustion, heroin relapse rats, neurotrophic factor, nerve cell ultrastructure, acupuncture, midbrain, Dazhui (DU14), Baihui (DU20), NSFC grant, neural regeneration
Introduction
Opioid drugs, such as heroin, are addictive and can produce physical and psychological dependence after use. Heroin abuse can damage many brain areas, including the pedunculopontine tegmental nucleus of the midbrain, the ventral tegmental area, and nucleus accumbens[1,2]. The ventral tegmental area contains dopamine neurons involved in the meso-limbic dopamine system, and along with the nucleus accumbens, are the main brain regions associated with the reward pathway[1]. Rodd et al.[3] found that local injection of 5-hydroxytryptamine receptor agonist in mouse ventral tegmental area could increase the activity of dopamine neurons. Interestingly, injection of 5-hydroxytryptamine receptor antagonist in the posterior lobe of the ventral tegmental area could suppress alcohol and cocaine addiction[3]. Our previous study demonstrated that neuronal degeneration and swelling were evident in the prefrontal lobe cortex and nucleus accumbens of rats with heroin relapse[4]. These results indicated that persistent use or one large-dose of heroin induced acute or chronic damage to the nervous system. The ventral tegmental area-nucleus accumbens pathway is a site where all addictive drugs produce acute reward effects[1]. Moreover, the ventral tegmental area is an essential region in the reward pathway, however our previous study neglected the ventral tegmental area. Thus, this study sought to observe the changes in neuronal ultrastructure in the ventral tegmental area during heroin relapse.
Heroin spongiform leukoencephalopathy is an organic brain disease occurring in white matter of the central nervous system from addicts who inhaled or injected heroin. This disease is characterized by the presence of spongy degeneration in the white matter[5,6,7,8,9]. Recurrent drug use after heroin addiction may cause the occurrence of heroin spongiform leukoencephalopathy. Our previous studies[4,10,11] concerning clinical and experimental research into heroin relapse verified that long-period large-dose use of heroin led to the alteration in brain morphology, such as changes in brain cell ultrastructure and brain imaging. Acupuncture treatment has been a feasible clinical intervention when heroin spongiform leukoencephalopathy occurs[12]. Acupuncture promotes the rehabilitation of brain injury, and protects and nourishes nerve cells in the central nervous system[13]. Nevertheless, at present, acupuncture has not been widely used to treat heroin addiction. Medicine, such as methadone, buprenorphine, and naltrexone, is still the first choice for detoxification in the clinic. Methadone, a typical μ opioid receptor agonist, lessens abstinence syndrome, and is extensively used in detoxification and maintenance therapy for heroin addiction in the clinic. Therefore, methadone is selected as a positive control. Brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor are strongly associated with neuronal growth, development, differentiation, maturity, nervous system development, neuronal injury, and some diseases in the nervous system[14,15,16,17,18]. Brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor participate in nerve repair, contribute to nervous system development, activate neural pathways, and protect and repair injured neurons[14,15,16,17,18]. A previous study suggested that acupuncture regulated neurotrophic factors after cerebral ischemia[12]. Following the occurrence of cerebral ischemia, apparently increased brain-derived neurotrophic factor protein expression significantly relieved ischemic brain injury (such as cerebral edema), and inhibited neuronal necrosis[19]. This study was designed to observe the structure of brain tissue, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor expression changes, and acupuncture effects in heroin relapse rats, and then to further investigate the precise mechanisms of acupuncture treatment for heroin-associated encephalopathy.
Results
Quantitative analysis of experimental animals
A total of 32 rats were randomly and equally divided into normal, model, acupuncture and methadone groups. A rat model of heroin relapse was established by narcotic use until addiction occurred, and then creating a detoxification process by withholding heroin from the model, acupuncture and methadone groups. Model rats in the acupuncture group received acupuncture at Baihui (DU20) and Dazhui (DU14) during the detoxification period. Rats in the methadone group were intragastrically administered a decreasing amount of methadone during the detoxification period. All rats were included in the final analysis.
Acupuncture improved neuronal ultrastructure of the ventral tegmental area in rats with heroin relapse
Electron microscopy showed clear nuclear membranes, normal rough endoplasmic reticulum, ribosomes and nuclei, abundant rough endoplasmic reticulum and mitochondria, and slightly expanded rough endoplasmic reticulum in the ventral tegmental area of rats in the normal group. In the model group, nuclear membrane boundaries were obscure, rough endoplasmic reticulum expansion was seen, along with vacuolar degeneration of some mitochondria in the ventral tegmental area, indicating that nerve cells were severely injured in rats with heroin relapse. In the methadone and acupuncture groups, nuclear membranes were visible, ribosomes and rough endoplasmic reticulum were abundant, rough endoplasmic reticulum was normal or lightly expanded, and a few mitochondria were vacuolar and degenerated in the ventral tegmental area. These results suggested that acupuncture protected nerve cells against injury in rats with heroin relapse. In addition, no major difference in neuronal ultrastructure was detected in the ventral tegmental area of heroin relapse rats between the methadone and acupuncture groups (Figure 1).
Figure 1.
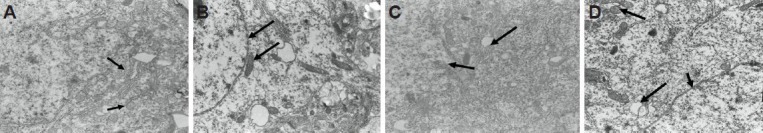
Acupuncture effects on the ultrastructure in the ventral tegmental area of heroin relapse rats (transmission electron microscopy, × 10,000).
(A) Normal group: normal ribosomes, mitochondria and rough endoplasmic reticulum in the ventral tegmental area. Arrows show endoplasmic reticulum. (B) Model group: reduced number of ribosomes, decreased number of mitochondria, partial vacuolar degeneration, and expanded rough endoplasmic reticulum. Arrows show nuclear membranes and mitochondria. (C) Methadone group: clear nuclear membranes, abundant ribosomes and rough endoplasmic reticulum, and a few vacuolar mitochondria. Arrows show vacuolar mitochondria and ribosomes. (D) Acupuncture group: clear nuclear membranes, abundant uniform ribosomes, a few vacuolar mitochondria, and lightly expanded rough endoplasmic reticulum. Arrows show nuclear membranes and mitochondria.
Acupuncture increased brain-derived neurotrophic factor expression in the ventral tegmental area of heroin relapse rats
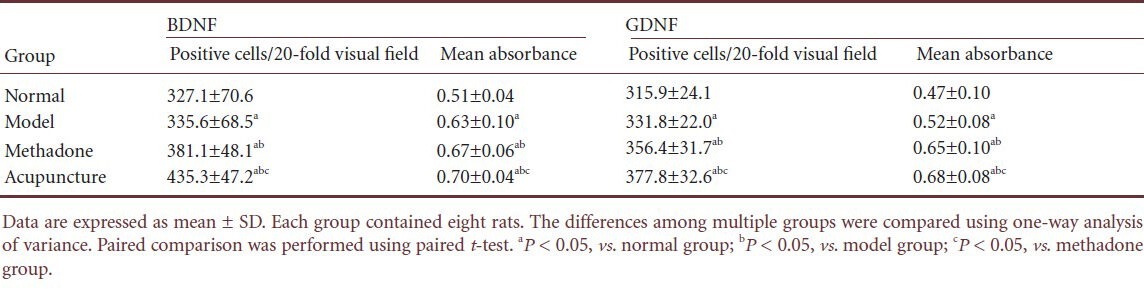
Immunohistochemical staining demonstrated that compared with the normal group, the number of brain-derived neurotrophic factor-positive cells and mean absorbance were greater in the ventral tegmental area of heroin relapse rats (P < 0.05). The number of brain-derived neurotrophic factor-positive cells and mean absorbance were increased after treatment with methadone or acupuncture (P < 0.05). The number of brain-derived neurotrophic factor-positive cells and mean absorbance were greater in the acupuncture group that those in the methadone group (P < 0.05; Figure 2, Table 1).
Figure 2.
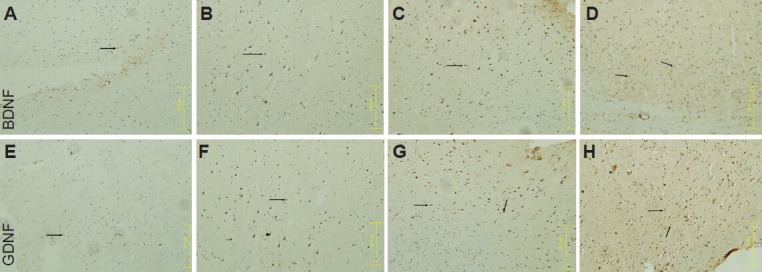
Acupuncture effects on brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) expression in the ventral tegmental area of heroin relapse rats (immunohistochemical staining, × 20).
Arrows show BDNF or GDNF expression. (A, E) Normal group: BDNF or GDNF expression was visible in the tissue. (B, F) Model group: BDNF or GDNF expression was increased after heroin relapse. In the methadone group (C, G) and acupuncture group (D, H), BDNF or GDNF expression was noticeably increased. Moreover, BDNF expression was greater in the acupuncture group than that in the methadone group.
Table 1.
Acupuncture effects on brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) expression in the ventral tegmental area of heroin relapse rats
Acupuncture increased glial cell line-derived neurotrophic factor expression in the ventral tegmental area of heroin relapse rats
Immunohistochemical staining showed that compared with the normal group, the number of glial cell line-derived neurotrophic factor-positive cells and mean absorbance were greater in the ventral tegmental area of heroin relapse rats (P < 0.05). The number of glial cell line-derived neurotrophic factor-positive cells and mean absorbance were increased after treatment with methadone or acupuncture (P < 0.05). The number of glial cell line-derived neurotrophic factor-positive cells and mean absorbance were greater in the acupuncture group compared with the methadone group (P < 0.05; Figure 2, Table 1).
Discussion
Acupuncture at Baihui and Dazhui normalizes neuronal ultrastructure in the ventral tegmental area of heroin relapse rats. Acupuncture at Baihui and Dazhui also contributes to brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor expression, and protects nerve cells against injury in heroin relapse rats. These results suggested that acupuncture had protective effects on nerve cells in the ventral tegmental area of heroin relapse rats at the levels of cell morphology and protein molecule expression.
Morphological alteration in injured brain induced by heroin and acupuncture
Numerous studies suggested that white matter injury and brain atrophy were detectable in the brain of heroin-dependent subjects[20,21,22,23,24,25,26,27,28,29,30]. Relevant imaging examination also demonstrated that abnormal signal changes were detected in the white matter, cerebellar white matter, and posterior limb of the internal capsule of these subjects. Other studies confirmed that ultrastructural alterations were visible in the cerebral cortex, hypothalamus and pituitary gland of heroin-addicted rats, monkeys and humans[30,31,32,33,34]. These findings suggest that extensive pathological lesions were detected in the brain of heroin-addicted rats, mainly present as neuronal degeneration and death, which gave a morphological reason behind the brain injury induced by relapse.
We have performed many studies on detoxification with acupuncture, and verified that acupuncture at Zusanli (ST36) could improve abstinence symptoms in morphine-addicted rats. Acupuncture lessened protracted abstinence symptoms in heroin-addicted persons during the rehabilitation period[35]. Acupuncture combined with psychological desensitization could improve sleep and anxiety disorders[36]. Magnetic resonance imaging and functional magnetic resonance imaging revealed that heroin could activate brain areas regulating craving-related reward, learning and memory, cognition and emotion, but acupuncture at Zusanli suppressed the activation of craving-related brain areas[10,11]. Experimental studies confirmed that acupuncture improved neuronal ultrastructure in the prefrontal lobe cortex, hippocampus and nucleus accumbens of heroin relapse rats[37,38,39]. These results indicated that acupuncture had a protective effect on the injured brain tissue of heroin relapse rats.
Given the findings described in the above studies, this study further continued research into the effect of heroin relapse on the brain by observing the alterations in ultrastructure of the ventral tegmental area in heroin relapse rats using electron microscopy. Our findings verified the morphological changes in injured brains after heroin relapse. After persistent treatment with acupuncture, the injury to nerve cells was improved, suggesting that acupuncture had a protective effect on injured nerve cells. The protective effects of acupuncture provide experimental evidence for clinical application of acupuncture.
Significance of studying the ventral tegmental area
The opioid-induced reward effect is a direct reason for opioid addiction[40]. A previous study showed that brain areas, such as the nucleus accumbens, ventral tegmental area, interior cortex of the frontal lobe, the pedunculopontine tegmental nucleus, hippocampus, hypothalamus, ventral globus pallidus and amygdale, were involved in the opioid-induced reward effect. These nuclear groups and their connecting structures were called the reward pathway[41].
There are two reward pathways involved in relapse addiction: the mesolimbic pathway (from the ventral tegmental area to the nucleus accumbens), and the mesocortical pathway (from the ventral tegmental area to the frontal cortex). The ventral tegmental area is an essential area in these two dopamine reward pathways, and contains numerous dopaminergic neurons. The release of dopamine from the ventral tegmental area produces the rewarding effect of heroin and induces abstinence symptoms during withdrawal. Thus, drug addicts experienced pleasant feelings and cognitive disorder. Therefore, the ventral tegmental area was selected as a reward pathway to observe morphological changes and the expression of neurotrophic factors under an electron microscope[41].
Owing to the complexity and integrity of the nervous system, various neurotransmitters, signal pathways and genes create a networked adjusting process during addiction and relapse. Studies addressing molecular mechanisms of addiction relapse mainly contained two aspects: (1) monoamine neurotransmitters and receptors such as dopamine, noradrenaline and 5-hydroxytryptamine. Most addictive drugs cause an increase in dopamine release in the nucleus accumbens dominated by dopaminergic neurons in the ventral tegmental area[42]. Dopamine acts on dopamine receptors and exerts an important effect on studies into learning and memory, especially when behaviors are related to reward. (2) Excitatory and inhibitory amino acids and their receptors. Glutamate and its receptors (mainly N-methyl-D-aspartate receptor and amino-3-hydroxy-5-isoxazolepropionic acid receptor) play an important role in neural plasticity of addiction. The nucleus accumbens is strongly associated with learning and memory-related structures such as the prefrontal cortex, hippocampus and amygdale, which possibly indirectly induce the formation of habitual memory via the dorsal striatum, regulates obsessive-compulsive behaviors and generates relapse[43,44,45,46,47,48,49,50,51]. The present study investigated molecular mechanisms of heroin relapse from the angle of neurotrophic factor expression.
Neurotrophic factor expression and acupuncture
Glial cell line-derived neurotrophic factor has been shown to protect neurons against injury, and also contributes to axon growth[52]. These effects occur because glial cell line-derived neurotrophic factor bound to its α1 receptor, and then activated receptor tyrosine kinase[53]. Glial cell line-derived neurotrophic factor is the only neurotrophic factor that resists neuronal apoptosis and blocks cell atrophy[54]. Glial cell line-derived neurotrophic factor can enhance cell proliferative ability in the subventricular zone and subgranular zone of the hippocampal dentate gyrus, and exogenous glial cell line-derived neurotrophic factor accelerates the repair of nerve injury in the central nervous system[55].
Brain-derived neurotrophic factor promotes the growth, development, differentiation[56] and functional expression of nerve cells during the development of the central nervous system. Simultaneously, brain-derived neurotrophic factor maintained neuronal function in the central nervous system. Brain-derived neurotrophic factor promoted synaptic plasticity, altered neuronal morphology in the brain, increased the density of synaptic terminals and contributed to the outgrowth of dendrites and axons[14]. Moreover, brain-derived neurotrophic factor suppressed nociceptive stimulus and led to neuronal regeneration. Heroin-associated encephalopathy was closely correlated to learning and memory. Drug addiction and learning and memory processes occur in common anatomic structures in the central nervous system. Glutamate and dopamine receptor systems and their interactions regulate the formation and consolidation of addiction memory[57]. Chronic drug addiction increases the expression levels of mature brain-derived neurotrophic factor in the ventral tegmental area, which was associated with the dopamine system and reward circuit[15]. Vargas-Perez et al.[16] suggested that mature brain-derived neurotrophic factor directly participated in the switching mechanism of the ventral tegmental area, and changed reward circuits from non-dopamine-dependent into dopamine-dependent pathways. Rats have displayed drug addiction and withdrawal behaviors after mature brain-derived neurotrophic factor was injected into neurons in the ventral tegmental area. Luo et al.[16] confirmed that acupuncture at Dazhui and Baihui enhanced brain-derived neurotrophic factor expression in rats with focal cerebral ischemia, blocked intracellular Ca2+ overloading, and stabilized the intracellular environment.
These studies demonstrated that neurotrophic factors prevented the death of injured neurons, regulated synaptic plasticity, reduced neural degeneration, prevented disease progression, stimulated axon growth and promoted regeneration[17,18]. This study showed that brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor expression was increased in the rat frontal lobe and ventral tegmental area of heroin relapse rats, and indicated that chronic toxic effects of heroin caused nerve cell injury, led to compensatory protection of the body, increased endogenous brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor expression, and repaired injured nerve cells. Results from this study verified that this kind of compensatory protection could not repair injured nerve cells, and its repair effects were far less than the nociceptive effects of chronic heroin. These findings were consistent with the results in the model group (brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor expression was increased and cell structure was obviously damaged).
In the present study, an increased brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor expression after acupuncture had repair effects on injured nerve cells, indicating that heroin relapse caused a compensatory increase in brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor expression in the ventral tegmental area, and acupuncture increased brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor expression. This result is helpful to explain why acupuncture can protect nerve cells against injury.
Knowledge of the effects of acupuncture in traditional Chinese medicine
Traditional Chinese medicine believes that Baihui is strongly associated with the brain, and acupuncture at Baihui can regulate cerebral functions. Acupuncture at Baihui induces consciousness-restoring resuscitation, calms the nerves, dredges Du meridian, and relieves epilepsy. Dazhui is the crossing point where the three Yang Meridians of the Hand and the Du meridian cross and converge. New Compilation of Acupuncture Points says that Dazhui is an essential point in the body, and acupuncture at Dazhui can release various nervous symptoms. Acupuncture at Baihui and Dazhui contributes to the recovery of nerve functions, increases brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor expression in the rat frontal lobe and ventral tegmental area. Taken together, acupuncture has a promoting effect on nerve regeneration, repair and plasticity in heroin relapse rats.
Materials and Methods
Design
A randomized controlled animal study.
Time and setting
Experiments were performed at the Institute of Acupuncture and Meridian, Anhui University of Chinese Medicine, China in January 2013.
Materials
Animals
A total of 32 (16 males, 16 females) clean, Wistar rats aged 4 months and weighing 200 ± 20 g were provided by the Experimental Animal Center, Nanjing Medical University, China (license No. SYXK (Su) 2008-0007). All rats were allowed free access to standard solid feed at 22 ± 2°C and maintained in relative humidity of 50–70%. Experimental disposals were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[58].
Drugs
Heroin, a derivative of diacetylmorphine, chemical formula C21H23NO5, purity 90%, was provided and identified by Anhui Provincial Narcotic Prohibition Office in China. Heroin was diluted by physiological saline. Methadone, chemical formula C21H27NO·HCl, preparation for oral use, specification 10 mg/10 mL, was purchased from Central Pharmaceutical Co., Ltd., Tianjin, China, Approval No. GYZZ H10970303, lot No. 090919.
Methods
Establishment of a rat model of heroin relapse
An increasing amount of heroin was injected into the left gastrocnemius muscle of the posterior lower limb for 8 consecutive days (as narcotics use). From day 1 to day 8, 0.8, 1.2, 1.6, 2.0, 2.4, 2.8, 3.2 and 3.6 mg heroin was injected daily. From day 1 to day 6, the injection was performed once a day. From day 7 to day 8, injection was conducted twice a day (1.6 mg once on day 7, and 1.8 mg once on day 8). The rats received heroin at 7:00 and 19:00. After using narcotics, intramuscular injection of heroin was terminated for 5 days. Withdrawal symptoms and conditioned place preference were used to judge whether the model was established. Natural withdrawal was considered as detoxification. The rat model of heroin relapse was established using the method of narcotics use (addiction) → detoxification, and the addiction and detoxification process was repeated three times[59]. The rats in the normal group were administered physiological saline during narcotics use, 0.2 mL/time.
Acupuncture treatment
The rats in the acupuncture group underwent acupuncture during the period of detoxification. The rats were gently placed in a tailor-made fixator. In accordance with the Acupuncture Atlas in Rats[60], acupuncture at Baihui (on the head, the midpoint of the anterior hairline, at the midpoint of the line connection the apexes of both ears) and Dazhui (on the posterior median line, in the depression below the spinous process of the 7th cervical vertebra) was conducted using a 25 mm stainless steel acupuncture needle (0.30 mm diameter, 25 mm long; Suzhou Medical Sino-foreign Joint Venture, Suzhou, Jiangsu Province, China) at an angle of 30° and with a depth of 12 mm. The needle was held in place for 30 minutes. Acupuncture was carried out once at 7:00 a.m. every day, for 5 consecutive days.
Intragastric administration of methadone
A decreasing amount of methadone was intragastrically given to the rats in the methadone group during the period of detoxification. From day 1 to day 4, the dose of methadone was respectively 0.4, 0.3, 0.2 and 0.1 mg.
Specimen collection and transmission electron microscopy for neuronal ultrastructure in the ventral tegmental area of rats
All rats were anesthetized with 10% chloral hydrate (0.36 mL/100 g) via intraperitoneal injection. After aortic cannulation, the right atrium was cut, perfused with 0.1 mol/L PBS 250 mL, and fixed with 4% paraformaldehyde + 0.1 mol/L PBS 150 mL for 30 minutes. In accordance with The Rat Brain in Stereotaxic Coordinates[61], the rats were decapitated, and the ventral tegmental area of one hemisphere was obtained and cut into 1 mm3 blocks. These blocks were fixed in a 2.5% glutaral for 24 hours, immersed in PBS for 1–6 hours, fixed in 1% osmic acid for 1–2 hours, sliced into 70 nm-thick sections, and observed with a transmission electron microscope (JEM-1230; JEOL, Tokyo, Japan).
Immunohistochemical staining for brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor expression in the ventral tegmental area of rats
The ventral tegmental area of another hemisphere was fixed in 4% paraformaldehyde, dehydrated in alcohol, permeabilized in xylene, embedded in paraffin, and then serially sliced into 4 μm-thick sections. These sections were incubated with rabbit anti-rat brain-derived neurotrophic factor monoclonal antibody (1:100; Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China) and rabbit anti-rat glial cell line-derived neurotrophic factor monoclonal antibody (1:100; Beijing Biosynthesis Biotechnology Co., Ltd.) in a wet box overnight at 4°C, followed by an incubation in biotinylated goat anti-rabbit IgG (Boster, Wuhan, Hubei Province, China) at room temperature for 20 minutes. Using DP801 morphology image analysis system (Jiangsu JEDA Science-Technology Development Co., Ltd., Nanjing, Jiangsu Province, China), three discontinuous sections of each rat were selected, and five nonoverlapping visual fields of each section were selected under a light microscope (× 20) for cell counting. Cells with brown particles in the cytoplasm were considered as positive cells. The number of positive cells in each visual field, and mean absorbance of each section were calculated.
Statistical analysis
Experimental results were expressed as mean ± SD, and processed with SPSS 13.0 software (SPSS, Chicago, IL, USA). The differences among multiple groups were compared using one-way analysis of variance. Paired comparison was performed using paired t-test. A value of P < 0.05 was considered statistically significant.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81173325; the Anhui Provincial Higher Learning School Excellent Youth Project, No. 2010SQRL105; Anhui Provincial Higher Learning Natural Science Project, No. KJ2013Z180.
Conflicts of interest: None declared.
Peer review: This study observed cell structure and neurotrophic factor changes of the ventral tegmental area of heroin relapse rats. Results verified that acupuncture at Baihui and Dazhui could protect neurons against injury in heroin relapse rats and confirmed the mechanisms of acupuncture effects on detoxification.
Copyedited by Apricò K, Bai WZ, Liang Y, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- [1].Zhang JD, Li WQ, Song XH, et al. Expressions of related genes in the shell of accumbens nuclei when constructing a rat model of chronic morphine-induced conditioned place aversion. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17(20):3687–3691. [Google Scholar]
- [2].Xu WC. The development of research on brain's reward system. Guangzhou Yiyao. 2008;39(2):1–2. [Google Scholar]
- [3].Rodd ZA, Gryszowka VE, Toalston JE, et al. The reinforcing actions of a serotonin-3 receptor agonist within the ventral tegmental area: evidence for subregional and genetic differences and involvement of dopamine neurons. J Pharmacol Exp Ther. 2007;321(3):1003–1012. doi: 10.1124/jpet.106.112607. [DOI] [PubMed] [Google Scholar]
- [4].Zhang RJ, Cai XH, Song XG. Effect of neuronal ultrastructures in the brains of heroin re-addicted rats. Zhongguo Yaowu Yilaixing Zazhi. 2008;17(6):424–426. [Google Scholar]
- [5].Chang WC, Lo CP, Kao HW, et al. MRI features of spongiform leukoencephalopathy following heroin inhalation. Neurology. 2006;67(3):504. doi: 10.1212/01.wnl.0000216155.52411.6e. [DOI] [PubMed] [Google Scholar]
- [6].Wu LH. Clinical analysis of 16 cases spongiform leukoencephalopathy caused by heroin. Liaoning Yixue Zazhi. 2009;23(5):235–237. [Google Scholar]
- [7].Yin J, Lu BX, Zhou L, et al. Pathological analysis of heroin spongiform leukoencephalopathy: 4 cases report. Disi Junyi Daxue Xuebao. 2007;28(15):1415–1417. [Google Scholar]
- [8].Halloran O, Ifthikharuddin S, Samkoff L. Leukoencephalopathy from “chasing the dragon”. Neurology. 2005;64(10):1755. doi: 10.1212/01.WNL.0000149907.63410.DA. [DOI] [PubMed] [Google Scholar]
- [9].Offiah C, Hall E. Heroin-induced leukoencephalopathy: characterization using MRI, diffusion-weighted imaging, and MR spectroscopy. Clin Radiol. 2008;63(2):146–152. doi: 10.1016/j.crad.2007.07.021. [DOI] [PubMed] [Google Scholar]
- [10].Song XG, Li CF, Huang JJ, et al. Effects of acupuncture on cue-induced heroin addicts by functional magnetic resonance. Anhui Zhongyi Xueyuan Xuebao. 2008;27(4):21–23. [Google Scholar]
- [11].Song XG, Li CF, Hu L, et al. Effect of acupuncture on heroin cue-induced functional magnetic resonance images in heroin-addicted human subjects. Zhen Ci Yan Jiu. 2011;36(2):121–127. [PubMed] [Google Scholar]
- [12].Lv H, Song XG. Role of acupuncture regulate neurotrophic factors. Gansu Zhongyi Xueyuan Xuebao. 2011;28(3):65–68. [Google Scholar]
- [13].Sze FK, Wong E, Yi X, et al. Does acupuncture have additional value to standard poststroke motor rehabilitation? Stroke. 2002;33(1):186–194. doi: 10.1161/hs0102.101815. [DOI] [PubMed] [Google Scholar]
- [14].Li PP, Zhao Y, Zhu F, et al. Current advances in pro-BDNF. Zhongguo Fayi Xue Zazhi. 2011;26(3):207–210. [Google Scholar]
- [15].Vargas-Perez H, Ting-A Kee R, Walton CH, et al. Ventral tegmental area BDNF induces an opiate-dependent-like reward state in naive rats. Science. 2009;324(5935):1732–1734. doi: 10.1126/science.1168501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Luo ZD, Luo ZK, XU NG, et al. Effect of electroacupuncture on brain-derived neurotrophic factor in rats with focal brain ischemia. Zhen Ci Yan Jiu. 2002;27(2):105–107. [Google Scholar]
- [17].Ohmiya M, Shudai T, Nitta A, et al. Brain-derived neurotrophic factor alters cell migration of particular progenitors in the developing mouse cerebral cortex. Neurosci Lett. 2002;317(1):21–24. doi: 10.1016/s0304-3940(01)02412-0. [DOI] [PubMed] [Google Scholar]
- [18].Xiao CH, Wang YQ, Liu HM, et al. Intrastriatal glial cell line-derived neurotrophic factors for protecting dopaminergic neurons in the substantia nigra of mice with Parkinson disease. Neural Regen Res. 2007;2(4):207–210. [Google Scholar]
- [19].Schäbitz WR, Berger C, Kollmar R, et al. Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke. 2004;35(4):992–997. doi: 10.1161/01.STR.0000119754.85848.0D. [DOI] [PubMed] [Google Scholar]
- [20].Li XY. The study on electroneurophysiology of addict with heroin. Xiandai Dianshengli Xue Zazhi. 2006;13(4):205–207. [Google Scholar]
- [21].Zhang JJ, hu CW, Dong HB, et al. MRI manifestations of brain in the heroin-dependent patients. Shiyong Fangshe Xue Zazhi. 2007;23(6):731–733. [Google Scholar]
- [22].Yin XP, Xia ZB, Wu XJ, et al. Heroin-induced spongiform leucoencephalopathy: three cases report with review of the literature. Jiujiang Yixue. 2007;22(1):7–11. [Google Scholar]
- [23].Tang L, Su D. Physiological pathological characters and appearance of DWI of toxic encephalopathy in heroin. Beihua Daxue Xuebao: Ziran Kexue Ban. 2007;8(5):407–408. [Google Scholar]
- [24].Wang XJ, Yang QW, Lin W. Clinical manifestations and image characteristics of heroin encephalopathy. Nao yu Shenjing Jibing Zazhi. 2008;16(2):111–112. [Google Scholar]
- [25].Lin RQ, Huang Y, Deng Q, et al. Analysis of influencing factors on heroin dependence accompanied with peripheral neuropathy. Zhongguo Yaowu Ylaixing Zazhi. 2010;19(4):273–276. [Google Scholar]
- [26].Zhong JY, Zhang HY, Liu HW, et al. EEG and transcranial Doppler analysis in the heroin dependent patients. Guangzhou Yiyao. 2010;41(2):4–6. [Google Scholar]
- [27].Ding ZS, Yu KL, Zhao C, et al. Comparative analysis on brain computerized tomography of patients with heroin dependence and rehabilitation treatments. Shiyong Linchuang Yiyao Zazhi. 2010;14(1):39–41. [Google Scholar]
- [28].Shi Z, Pan SL, Zhou L, et al. MRI features of patients with heroin spongiform leukoencephaiopathy of different clinical stages. Zhonghua Fang She Xue Za Zhi. 2007;41(7):691–693. [Google Scholar]
- [29].Chen GB, Wei LL, Chen QH, et al. Image features and therapy of heroin spongiform leucoencephalopathy. Zhongguo Yiliao Qianyan. 2007;2(7):61–63. [Google Scholar]
- [30].Ouyang KX, Dong YC, Huang Z, et al. MRI diagnostic features of heroin spongiform leukoencephalopathy. Dangdai Yixue. 2010;16(36):1–2. [Google Scholar]
- [31].Leri F, Stewart J. The consequences of different “lapses” on relapse to heroin seeking in rats. Exp Clin Psychopharmacol. 2002;10(4):339–349. doi: 10.1037//1064-1297.10.4.339. [DOI] [PubMed] [Google Scholar]
- [32].Kiyatkin EA, Rebec GV. Impulse activity of ventral tegmental area neurons during heroin self-administration in rats. Neuroscience. 2001;102(3):565–580. doi: 10.1016/s0306-4522(00)00492-9. [DOI] [PubMed] [Google Scholar]
- [33].Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- [34].Ye J, Wei XL, Wei SX, et al. Changes of nervous tissue ultrastructures and neurotransmitter in brain of heroin re-addicted rats. Jiepou Xue Zazhi. 2004;27(6):331–333. [Google Scholar]
- [35].Song XG, Zhang J, Wang ZH, et al. Clinical observation on acupuncture combined with methadone for improving heroin withdrawal syndrome. Zhongguo Zhen Jiu. 2002;22(12):795–797. [Google Scholar]
- [36].Song XG, Hu L, Wang J, et al. Clinical observation on the effects of acupuncture combined with psychological desensitization therapy for sleep disorders and anxiety symptom of heroin-addicts. Zhongguo Yaowu Yilaixing Zazhi. 2010;19(4):269–272. [Google Scholar]
- [37].Song XG, Zhang Y, Zhu YL, et al. Effects of acupuncture Zusanli on conditioned place preference in rats with morphine dependence. Anhui Zhongyi Xueyuan Xuebao. 2007;26(2):21–23. [Google Scholar]
- [38].Cai XH, Shi FZ, Song XG, et al. Effects of acupuncture and moxibustion on levels of dopamine and 5-hydroxytryptamine in brains of rats with heroin relapse. Anhui Zhongyi Xueyuan Xuebao. 2011;30(3):38–40. [Google Scholar]
- [39].Zhang RJ, Song XG, Cai XH. Influence of acupuncture and moxibustion on conditional position preference and prefrontal cortical ultrastructure in heroin re-addicted rats. Zhen Ci Yan Jiu. 2009;34(2):97–100. [PubMed] [Google Scholar]
- [40].Sheng R, Gu ZL. Progress in the mechanisms and treatment of opiate dependence. Zhongguo Yesheng Zhiwu Ziyuan. 2002;21(1):5–8. [Google Scholar]
- [41].Wang F, Luo F, Han JS. Participation brain areas of opioid rewarding effects and its fiber projection. Shenjing Jiepou Xue Zazhi. 2000;16(3):273–276. [Google Scholar]
- [42].Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36(2-3):129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- [43].Wise RA. Addiction becomes a brain disease. Neuron. 2000;26(1):27–33. doi: 10.1016/s0896-6273(00)81134-4. [DOI] [PubMed] [Google Scholar]
- [44].Han JS. Beijing: Beijing Medical University Press; 1999. Principles of Neural Science. [Google Scholar]
- [45].Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25(3):515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- [46].Nestler EJ. Neurobiology. Total recall-the memory of addiction. Science. 2001;292(5525):2266–2267. doi: 10.1126/science.1063024. [DOI] [PubMed] [Google Scholar]
- [47].Beninger RJ, Miller R. Dopamine D1-like receptors and reward-related incentive learning. Neurosci Biobehav Rev. 1998;22(2):335–345. doi: 10.1016/s0149-7634(97)00019-5. [DOI] [PubMed] [Google Scholar]
- [48].Boening JA. Neurobiology of an addiction memory. J Neural Transm. 2001;108(6):755–765. doi: 10.1007/s007020170050. [DOI] [PubMed] [Google Scholar]
- [49].Vann SD, Brown MW, Erichsen JT, et al. Fos imaging reveals differential patterns of hippocampal and parahippocampal subfield activation in rats in response to different spatial memory tests. J Neurosci. 2000;20(7):2711–2718. doi: 10.1523/JNEUROSCI.20-07-02711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Xiao L, Sui N. Involvement of learning and memory in the development of addictive be-havior and the related brain mechanism. Zhongguo Shenjing Kexue Zazhi. 2003;19(1):50–53. 58. [Google Scholar]
- [51].Chen XL, Liu JG, Chi ZQ. Molecular and cellular mechanisms of opiate addiction. Zhonguo Yaoli Xue Tongbao. 2005;21(8):901–904. [Google Scholar]
- [52].Espejo M, Cutillas B, Arenas TE, et al. Increased survival of dopaminergic neurons in striatal grafts of fetal ventral mesencephalic cells exposed to neurotrophin-3 or glial cell line-derived neurotrophic factor. Cell Transplant. 2000;9(1):45–53. doi: 10.1177/096368970000900107. [DOI] [PubMed] [Google Scholar]
- [53].Tomac AC, Grinberg A, Huang SP, et al. Glial cell line-derived neurotrophic factor receptor alpha1 availability regulates glial cell line-derived neurotrophic factor signaling: evidence from mice carrying one or two mutated alleles. Neuroscience. 2000;95(4):1011–1023. doi: 10.1016/s0306-4522(99)00503-5. [DOI] [PubMed] [Google Scholar]
- [54].Gao MY, Xiao JD, ZHeng QX, et al. Differentiation traits of transgenic NSC with exogenetic expression of GDNF. Zhonghua Chuangshang Guke Zazhi. 2005;7(11):1067–1072. [Google Scholar]
- [55].Li YT, Jin GR, Xu HR, et al. The effect of GDNF on the proliferating cells of SVZ and SGZ as well as the recovery of learning and memory in adult rats after focal cerebral ischemia. Zhongfeng yu Shenjing Jibing Zazhi. 2005;22(2):111–115. [Google Scholar]
- [56].Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2(1):24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- [57].Ikemoto S. Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory. Neurosci Biobehav Rev. 2010;35(2):129–150. doi: 10.1016/j.neubiorev.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [59].Wei XL, Ye J, Zheng Y. Establishment of the model of heroin readdicted rats. Guangxi Yixue. 2004;26(6):783–785. [Google Scholar]
- [60].Hua XB. Acupuncture atlas in rats. Shiyan Dongwu yu Dongwu Shiyan. 1991;1:1–5. [Google Scholar]
- [61].Bao XM, Shu SY. Beijing: People's Medical Publishing House; 1991. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]


